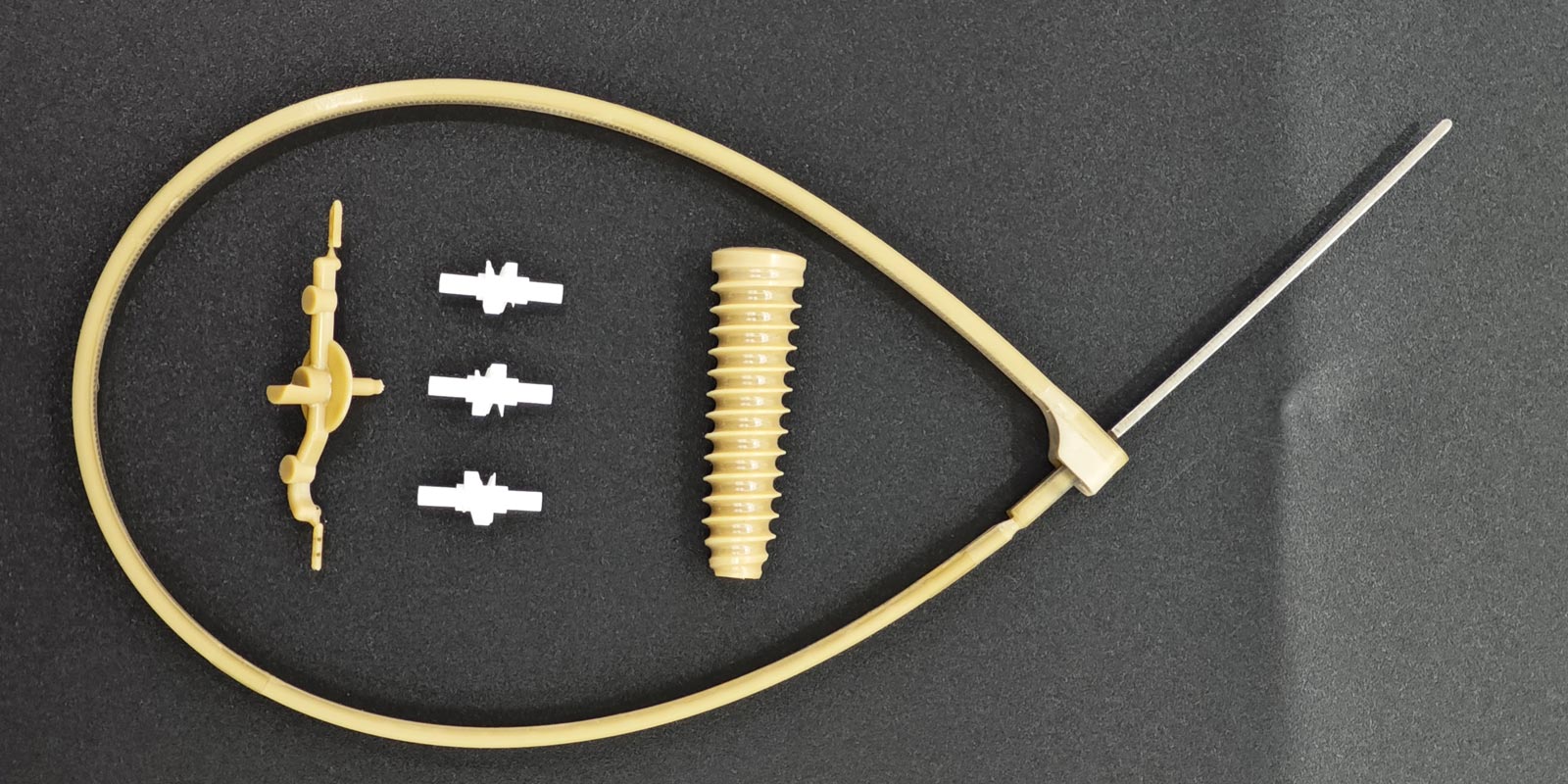
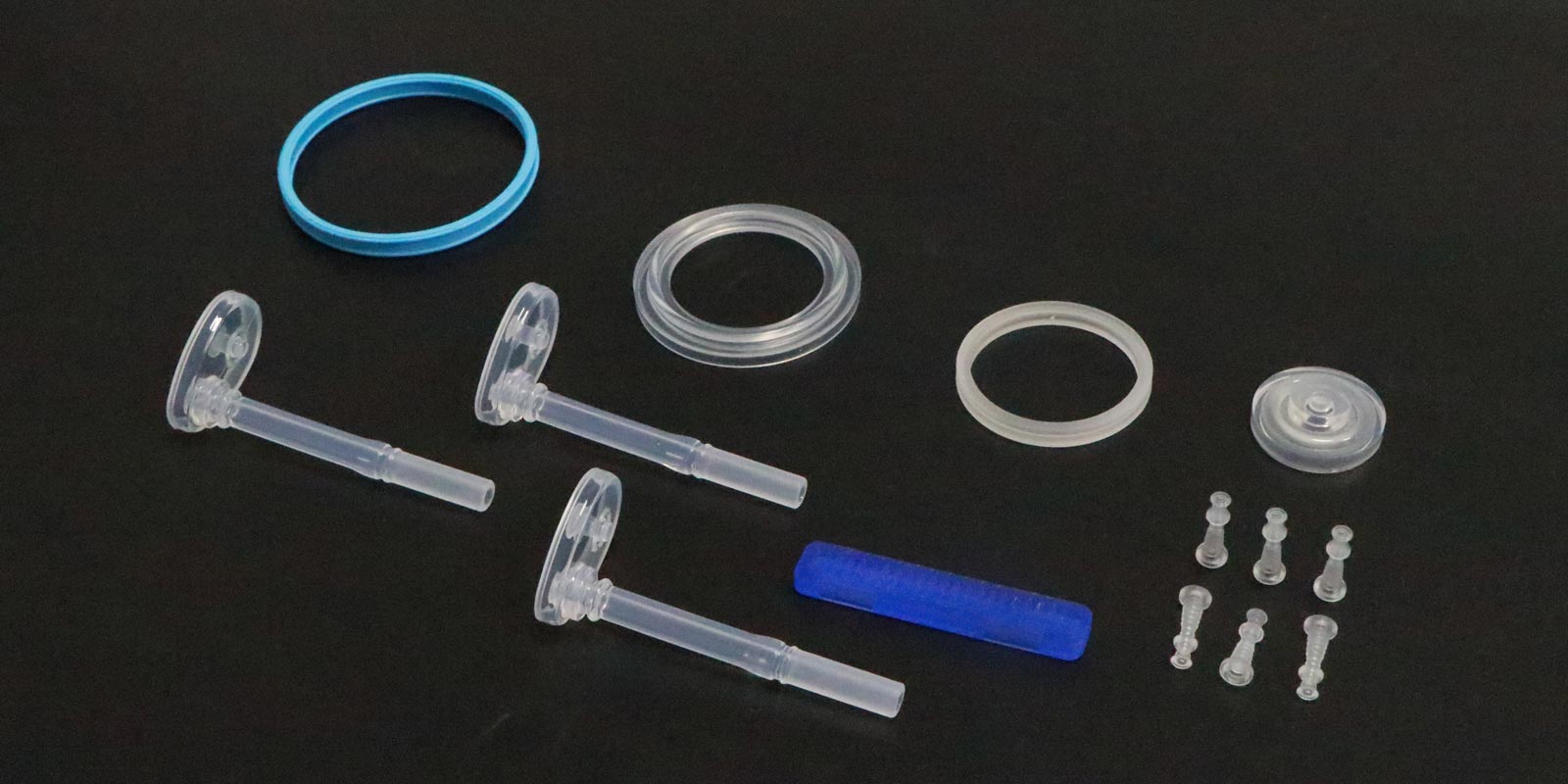
In the medical industry, injection molding technology is widely used in the manufacturing of various medical devices and consumables due to its high precision, high efficiency, and ability to produce products with complex structures. However, during the production process of medical injection-molded products, a whitening phenomenon occasionally occurs. This not only affects the appearance quality of the products but also raises concerns about their performance and safety. So, what exactly causes the whitening of medical injection-molded products? This article will delve into the scientific principles behind this phenomenon and propose corresponding solutions.
I. Definition and Manifestation of the Whitening Phenomenon
Whitening refers to the appearance of white spots, streaks, or an overall whitish tint on the surface or inside of injection-molded products. This anomaly not only reduces the aesthetic appeal of the products but, in severe cases, may also impact their mechanical properties, transparency, and biocompatibility, posing potential threats to the safety and effectiveness of medical products.

II. Main Causes of Whitening
1. Material Factors
-
High Moisture Content: Medical-grade plastic raw materials such as polypropylene (PP) and polycarbonate (PC) are hygroscopic. If not stored properly or dried sufficiently before injection molding, the moisture can evaporate at high temperatures, forming bubbles that leave tiny voids upon cooling, resulting in whitening on the surface or inside the product.
-
Incompatibility of Additives: To improve the processing performance or impart specific functions, additives such as plasticizers, stabilizers, and colorants are often added. If the additives are improperly selected or incompatible with the base material, they may precipitate during processing, forming white spots.
2. Improper Process Parameter Settings
-
Excessive Injection Speed: An excessively fast injection speed can cause severe shear in the melt within the mold cavity, generating a large amount of heat. This can lead to local overheating, material decomposition, or carbonization, resulting in black spots or whitening.
-
Improper Mold Temperature Control: If the mold temperature is too low, the melt cools too rapidly, increasing internal stress and leading to surface stress cracking or whitening. Conversely, if the mold temperature is too high, it can prolong the cooling time, increase the risk of product deformation, and indirectly affect the appearance quality.
-
Insufficient Packing Pressure and Time: Insufficient packing pressure and time can prevent the melt from fully filling the mold cavity, resulting in voids or uneven shrinkage inside the product, which manifests as whitening or sink marks on the surface.
3. Mold Design and Maintenance Issues
-
Mold Design Defects: Improper runner design or inadequate venting can cause air to be entrapped in the melt during filling, forming bubbles that appear as whitening upon cooling.
-
Mold Wear or Contamination: Over time, the mold surface may wear or accumulate dirt, affecting the flowability and filling effect of the melt and leading to a decline in the surface quality of the product, including whitening.
III. Solutions and Preventive Measures
1. Optimize Material Management
-
Ensure that raw materials are stored in a dry environment and dried sufficiently before use to control the moisture content within a reasonable range.
-
Select additives that are compatible with the base material to avoid substances that may cause chemical reactions or precipitation.
2. Fine-Tune Process Parameters
-
Set key parameters such as injection speed, mold temperature, packing pressure, and time reasonably based on the material characteristics and product requirements.
-
Implement multi-stage injection and packing to reduce melt shear heat and internal stress.
3. Improve Mold Design and Maintenance
-
Optimize the mold runner and venting system design to ensure smooth melt filling and minimize bubble formation.
-
Regularly inspect and clean the mold, and promptly repair worn parts to maintain the mold surface finish.
4. Strengthen Quality Control and Inspection
-
Establish a strict quality control system to conduct multi-stage inspections of raw materials, semi-finished products, and finished products, promptly identifying and addressing whitening issues.
-
Utilize advanced non-destructive testing techniques such as X-ray and ultrasonic testing to evaluate the internal structure of the product and ensure quality.
Conclusion
The whitening issue in medical injection-molded products is a complex engineering challenge that involves multiple fields such as material science, process control, and mold design. By gaining a deep understanding of the causes of whitening and implementing targeted preventive and corrective measures, we can effectively improve the quality and reliability of products, contributing to the healthy development of the medical industry. As manufacturers and researchers, we should continuously explore and innovate, constantly optimizing production processes to ensure that every medical injection-molded product meets the highest safety standards and quality requirements.











 Home
Home
