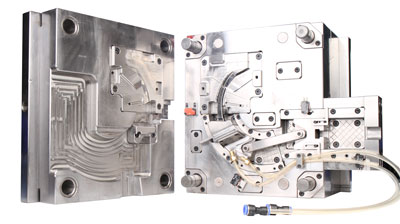
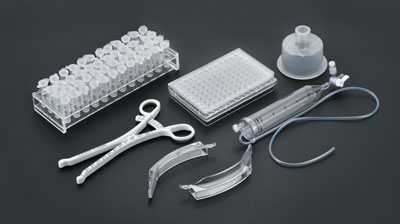
In the field of medical device manufacturing, injection molds serve as the core production tools, with their performance directly determining the accuracy, reliability, and safety of the products. Medical injection molds must meet stringent standards such as biocompatibility, chemical corrosion resistance, and high precision. The design and manufacturing of their core components have become pivotal for technological breakthroughs in the industry. This article delves into the core components of medical injection molds from the perspectives of functional classification and practical applications, analyzing their technical key points.
I. Molding System: The Core of Shaping Product Forms
-
Cavity and Core
The cavity and core are components directly involved in plastic molding, with their surface roughness required to be below Ra0.2μm to ensure the cleanliness of medical products. For example, in the injection molding of PPSU (polyphenylsulfone) dialysis housing, the fit accuracy between the cavity and core must be controlled within ±0.02mm to avoid sealing failures or functional defects caused by dimensional deviations.
-
Side Action Mechanisms
For products with complex structures, such as the multi-angle joints of surgical forceps or the buckle designs of respiratory masks, side action mechanisms achieve precise molding of side features through the cooperation of sliders and angle pins. These mechanisms must possess high wear resistance and positioning accuracy to withstand the impact of frequent opening and closing cycles.
-
Threaded Cores and Rings
In the manufacturing of infusion pump components or dialysis tube connectors, threaded cores and rings form internal/external thread structures through rotational motion. These components require hard alloy materials and precision grinding to ensure dimensional stability and interchangeability of threads.
II. Gating System: Precise Control of Melt Flow
-
Hot Runner Systems
Hot runner systems maintain the melt temperature through heating elements, reducing material waste and enhancing molding efficiency. In the injection molding of PEEK (polyetheretherketone) surgical instrument handles, hot runner systems must withstand temperatures above 300℃ and employ valve-gated designs to avoid weld lines.
-
Gate and Runner Designs
The position of gates and the dimensions of runners directly influence the filling effect of the melt. For example, in the injection molding of dental impression trays, sub-gates reduce gate marks on product surfaces, while optimizing runner diameters and lengths shortens filling times to under 15 seconds.
-
Venting Systems
Vents, typically placed at the ends of cavities or on parting lines, expel gases from the melt. In the injection molding of miniature medical sensor housings, vent slots must be controlled below 0.03mm in width to avoid surface defects caused by inadequate venting.
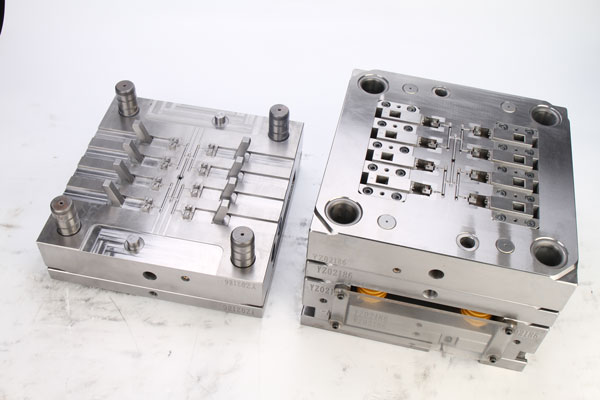
III. Temperature Control System: Ensuring Stable Molding
-
Cooling Circuit Layouts
Cooling circuits must be customized based on product shapes and wall thicknesses. For instance, in the injection molding of blood dialysis housing, conformal cooling water channels increase cooling efficiency by 40% while reducing warpage caused by temperature gradients.
-
Heating Elements and Insulation Layers
For high-temperature plastics like PEEK, molds require electric heating plates or hot oil circulation systems to maintain mold temperatures between 180℃ and 220℃. Insulation boards reduce heat loss and energy consumption.
IV. Ejection System: Ensuring Complete Product Demolding
-
Ejector Pins and Plates
Ejector pins must possess high strength and wear resistance to withstand frequent ejection cycles. In the injection molding of respiratory masks, a combination of flat-head ejector pins and ejector plates avoids deformation caused by uneven ejection forces.
-
Return Mechanisms
Return pins and springs ensure precise resetting of the ejection system during mold closing. In automated production lines, resetting accuracy must be controlled within 0.01mm to prevent mold damage caused by resetting errors.
V. Guiding and Positioning Systems: Ensuring Mold Precision
-
Guide Pins and Bushings
The clearance between guide pins and bushings must be controlled within 0.005mm–0.01mm to withstand high-frequency opening and closing cycles. In the precision injection molding of cardiac stent delivery systems, ball-guided pins further enhance guiding accuracy.
-
Locating Pins and Mold Clamping Devices
Locating pins achieve precise mold positioning through tapered fits, while mold clamping devices provide sufficient clamping forces to prevent melt leakage. In the injection molding of high-pressure sterilization equipment housing, clamping forces must exceed 500 tons.
VI. Auxiliary Systems: Enhancing Production Efficiency and Safety
-
Protective Covers and Magnetic Positioning
Protective covers prevent external contaminants from entering the mold, while magnetic positioning technologies achieve rapid mold alignment through electromagnetic forces. In the injection molding of multi-cavity molds, magnetic positioning reduces mold changeover times to under 5 minutes.
-
Packing Rings and Seals
Packing rings fill mold gaps to reduce material leakage, while rubber seals must possess high-temperature and chemical corrosion resistance. In the injection molding of PPSU materials, seals must withstand temperatures ranging from -40℃ to 180℃.
Conclusion
The design of core components in medical injection molds represents a deep integration of materials science, mechanical engineering, and precision manufacturing. From microscopic cavity processing to intelligent temperature control systems, every detail affects the safety and efficacy of medical products. With the continuous development of new materials and processes, medical injection molds are advancing toward higher precision, efficiency, and intelligence, providing robust technical support for global healthcare.











 Home
Home
