Medical molds, as the core process equipment for medical device manufacturing, directly determine the safety, effectiveness, and reliability of medical products. Compared with ordinary industrial molds, medical mold processing exhibits five core characteristics: high-precision requirements, strict quality control, complex process flows, material specificity, and intelligent trends. These characteristics collectively establish the technical barriers and industry thresholds for medical mold processing.
1. High-Precision Requirements: Micron-Level Tolerance Control in Precision Manufacturing
The precision requirements for medical molds far exceed those of ordinary industrial molds, with dimensional tolerances typically controlled within ±0.005mm, and some critical components even requiring ±0.001mm micron-level accuracy. For example, the mold cavity size deviation of a pacemaker housing must be strictly controlled within 0.002mm to ensure its sealing and biocompatibility after implantation in the human body. The curved surface precision of an artificial joint mold must simulate the natural morphology of human bones, with errors exceeding 0.01mm potentially leading to joint movement obstruction or accelerated wear.
To achieve this precision, medical mold processing extensively employs five-axis machining centers, slow-wire electrical discharge machining (EDM), and precision grinding and other high-end equipment. For instance, five-axis machining centers enable multi-axis coordinated motion for high-precision processing of complex curved surfaces, avoiding secondary clamping errors in traditional three-axis machining and elevating processing precision to the 0.003mm level. Additionally, the mold cavity surface must undergo multiple processes such as rough polishing, fine polishing, and mirror polishing, ultimately achieving a mirror finish of Ra0.02μm to reduce friction resistance during plastic molding and ensure product surface smoothness meets medical standards.
2. Strict Quality Control: Full Lifecycle Traceability and Multi-Layer Verification Systems
Medical mold processing must adhere to the ISO 13485 medical device quality management system, establishing a full lifecycle traceability system from design and processing to maintenance. For example, molds used for manufacturing implantable medical devices must record processing parameters, operator information, and inspection data for each process, enabling bidirectional traceability between the mold and the product through QR codes or RFID tags. If wear issues arise in a batch of joint implants processed by a specific mold, the root cause can be quickly traced to the mold's processing records, analyzing whether it resulted from improper steel heat treatment or polishing process defects.
In the verification phase, medical molds must pass multiple tests, including dimensional accuracy tests, wear resistance tests, UV resistance tests, and biocompatibility tests. For instance, in dimensional accuracy tests, a coordinate measuring machine (CMM) is used to perform full-size scanning of the mold cavity, generating a 3D data model for comparison with the original design drawings. Any error exceeding 0.005mm results in non-conformance. For mold components in contact with the human body, cytotoxicity tests and skin irritation tests and other biocompatibility tests are also required to ensure the material does not trigger allergies or inflammation.
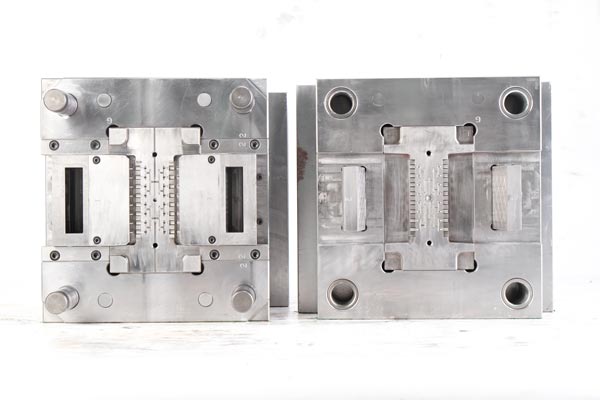
3. Complex Process Flows: Precision Molding Through Multi-Process Collaboration
The medical mold processing workflow encompasses design, material preparation, cutting, molding, heat treatment, polishing, assembly, mold testing, and debugging—nine key stages, each requiring strict control. Taking syringe mold processing as an example:
-
Design Phase: The mold cavity structure, flow channel system, and cooling water channel layout must be designed based on the syringe's capacity, scale accuracy, plunger movement smoothness, and other requirements. For instance, to prevent scale displacement due to mold shrinkage in syringes, micron-level compensation structures must be designed on the mold cavity surface, with shrinkage rates optimized through simulation software.
-
Processing Phase: High-speed milling is used to machine the mold core with a feed rate of 0.001mm, gradually cutting complex curved surfaces. Electrical discharge machining (EDM) is employed for fine hole erosion in the mold, ensuring hole diameter accuracy reaches 0.002mm.
-
Heat Treatment Phase: The mold steel undergoes vacuum quenching to elevate hardness to HRC52-55 while controlling deformation within 0.005mm to avoid dimensional overruns caused by heat treatment.
-
Mold Testing Phase: The mold is installed on an injection molding machine, and medical-grade polypropylene (PP) is used for trial production. The syringe's sealing, plunger resistance, and scale clarity are inspected. If issues such as flash, shrinkage, or deformation arise in the trial-molded products, adjustments to mold clamping force, injection pressure, or cooling time are made until the product pass rate reaches 99.9% or higher.
4. Material Specificity: Dual Considerations of Biocompatibility and Functionality
Medical mold materials must simultaneously meet requirements for biocompatibility, corrosion resistance, wear resistance, and high-temperature resistance. For example:
-
Implantable molds: Often use medical-grade stainless steel (e.g., 316LVM) or titanium alloys (e.g., Ti6Al4V), which offer excellent corrosion resistance and biocompatibility, preventing metal ion release or tissue reactions after implantation.
-
Disposable medical product molds: Frequently utilize polycarbonate (PC), polypropylene (PP), or polyethylene (PE) thermoplastics. These materials are cost-effective, easy to process, and can be enhanced with additives such as heat stabilizers, antioxidants, and UV absorbers. For instance, to manufacture high-temperature-resistant surgical instrument handles, 20% glass fiber can be added to PC, raising the heat deformation temperature from 130°C to 180°C.
-
Special-function molds: For example, when manufacturing sustained-release tablet molds, multi-layer co-injection molding technology is employed. Special flow channel designs in the mold enable layered injection of different drug components, forming core and sustained-release layers for precise drug delivery.
5. Intelligent Trends: Digital Technologies Empowering Precision Manufacturing
With the advancement of Industry 4.0 and smart manufacturing, medical mold processing is accelerating its transition toward intelligence. For example:
-
CAD/CAM Integrated Design: Three-dimensional mold models are created using computer-aided design (CAD) software, and computer-aided manufacturing (CAM) software generates machining paths, enabling seamless design-to-manufacturing transitions and reducing human errors.
-
3D Printing Technology: Used for rapid prototyping or customized mold manufacturing. For instance, when customizing a prosthetic mold for a patient with a rare condition, 3D scanning can capture residual limb data, and the mold can be directly printed to perfectly fit the limb, shortening development cycles from weeks to days.
-
Intelligent Monitoring Systems: Sensors installed during mold processing enable real-time monitoring of parameters such as cutting force, temperature, and vibration. Data analysis can predict mold lifespan or detect processing defects in advance. For example, when cutting force suddenly increases, the system can automatically adjust the feed rate to prevent tool breakage or mold deformation.
Conclusion: Precision Manufacturing Safeguarding Human Health
Medical mold processing is a "hidden yet critical" link in the medical industry chain, with its high precision, strict standards, complex processes, and material specificity forming the first line of defense for medical product safety. As medical technologies advance, medical mold processing is evolving toward higher precision, greater intelligence, and increased sustainability, providing more reliable manufacturing support for human health. From the minuscule housing of a pacemaker to the precisely curved surface of an artificial joint, every medical mold embodies precision manufacturing's commitment to safeguarding human dignity.
















 Home
Home
