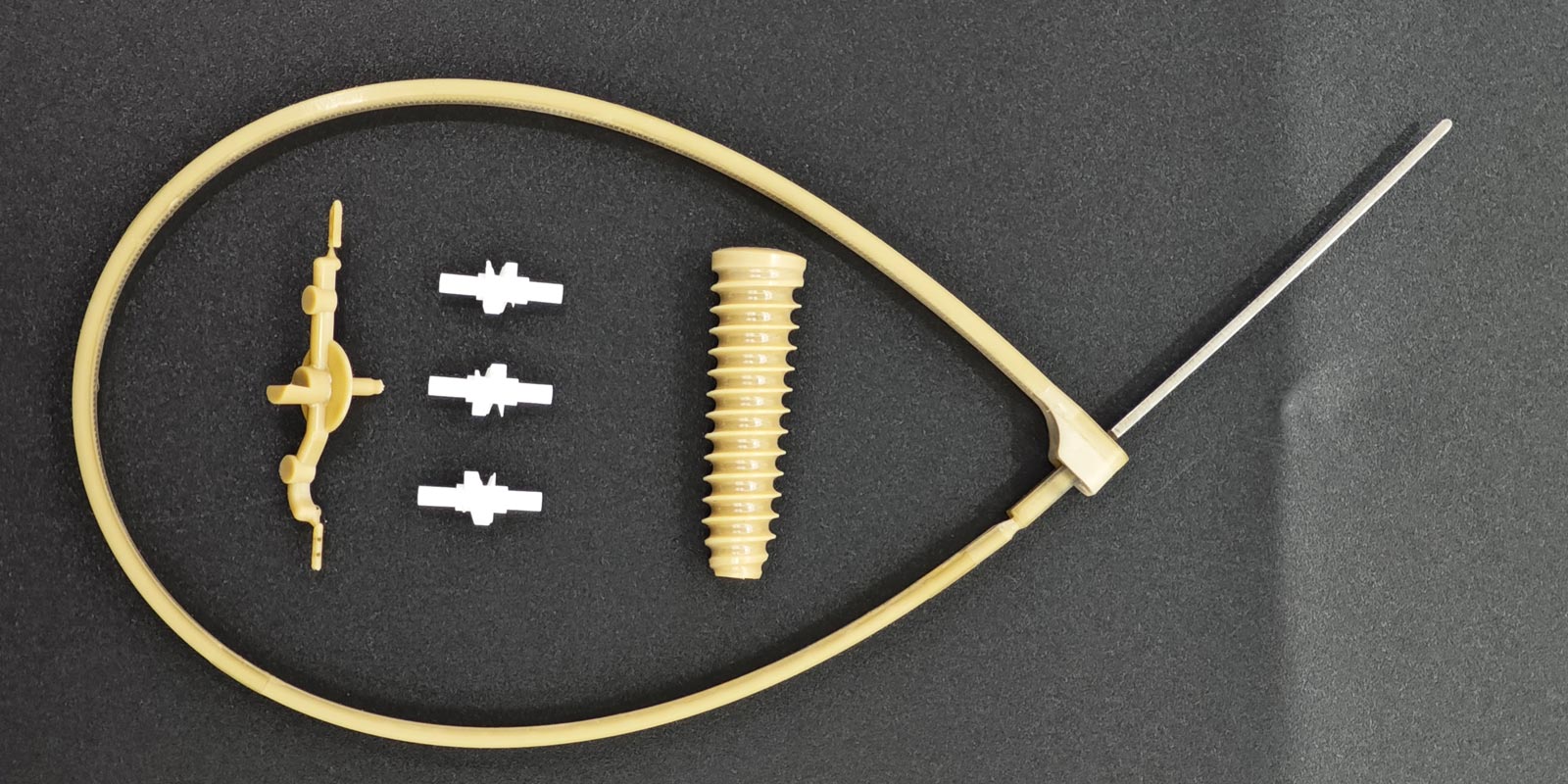
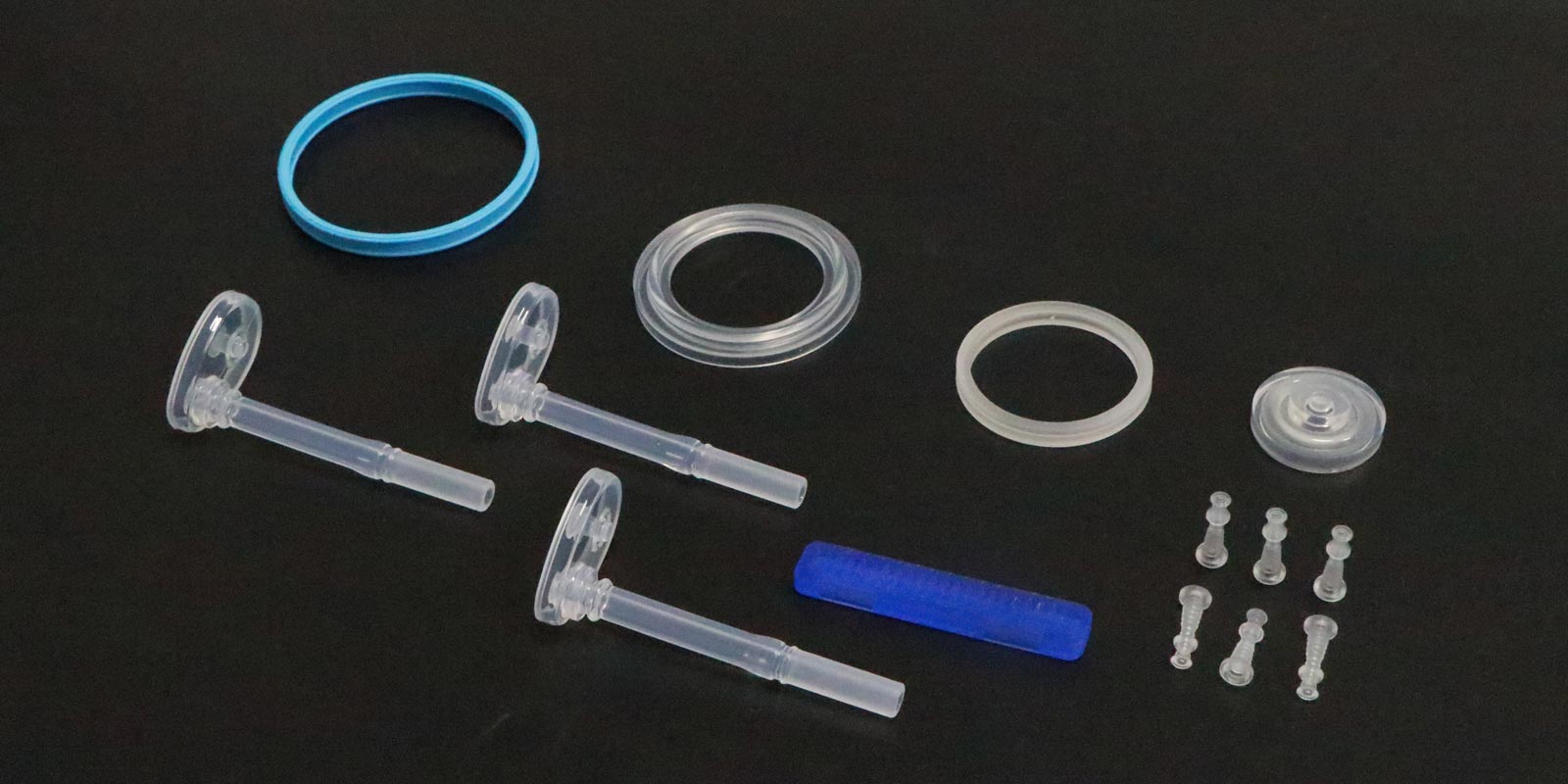
In the field of medical injection molding, burr problems not only affect product appearance but may also lead to seal failures, increased cleaning difficulties, and even patient safety risks. This article systematically outlines solutions to burr issues, covering aspects from mold design, process control, material selection, to post-processing techniques, all tailored to the characteristics of the medical industry.
I. Mold Design: Eliminating Burr Risks at the Source
-
Precision Optimization of Parting Surfaces
Medical products have extremely high requirements for surface finish. The parting surfaces need to be precisely machined using CNC technology, combined with EDM (Electrical Discharge Machining) to ensure a gap of ≤0.005mm. For complex structures, a multi-stage parting design is recommended. For example, in the production of a cardiac stent delivery system, a three-stage parting design reduced the burr incidence rate by 82%.
-
Innovation in Gating Systems
For high-flow medical-grade PC/ABS materials, it is advisable to use a submarine gate combined with a hot runner system. A case study in the production of a dialyzer housing showed that by reducing the gate diameter from φ1.2mm to φ0.8mm and controlling the hot runner temperature at 220℃, the flash thickness decreased from 0.15mm to 0.03mm.
-
Innovative Venting Structures
Medical products often contain fine structures, and traditional venting slots are prone to causing melt overflow. It is recommended to use a vacuum venting system that can maintain a venting precision of 0.02mm even when the cavity pressure reaches 150MPa. In the production of an insulin pen needle holder, this technology reduced the burr incidence rate from 12% to 0.3%.

II. Process Control: Precise Parameter Matching with Medical Material Properties
-
Multi-Stage Injection Strategy
Medical-grade PPSU materials require a five-stage injection process:
-
First stage: 80% speed for filling the runner
-
Second stage: 50% speed for filling the gate
-
Third stage: 30% speed for filling the main body
-
Fourth stage: 10% speed for holding pressure
-
Fifth stage: 5% speed for compensation shrinkage
A case study in the production of a surgical instrument handle showed that this process reduced the burr height from 0.2mm to 0.05mm.
-
Dynamic Holding Pressure Control
For high-rigidity materials like LCP, it is recommended to use pressure decay compensation technology. When the cavity pressure drops to 80% of the set value, the system automatically initiates secondary holding pressure with a pressure gradient controlled at 0.5MPa/s. In the production of an endoscope lens holder, this technology reduced the burr incidence rate from 18% to 2%.
-
Mold Temperature Gradient Management
Medical-grade PEEK materials require the establishment of a three-dimensional temperature field:
-
Gate area: 180℃ (±2℃)
-
Main body area: 160℃ (±1℃)
-
Edge area: 140℃ (±0.5℃)
In the production of an orthopedic implant mold, this technology reduced the burr width from 0.3mm to 0.08mm.
III. Material Selection: Matching Performance with Medical-Grade Specialized Materials
-
Low-Flow Modified Materials
For thin-walled medical packaging, it is recommended to use glass fiber-reinforced PP + 30% talc composite material, with a melt flow rate (MFR) controlled at 8-12g/10min. A case study in the production of blood collection tube caps showed that this material reduced the burr incidence rate from 25% to 5%.
-
Application of Self-Lubricating Additives
Adding 0.5% molybdenum disulfide to medical-grade POM can reduce the friction coefficient from 0.22 to 0.15. In the production of syringe pistons, this technology reduced the burr height from 0.15mm to 0.03mm.
-
Nano-Filling Technology
Adding 2% nano-silica to medical-grade PC can reduce the shrinkage rate from 0.6% to 0.3%. A case study in the production of a hemodialyzer housing showed that this technology reduced the burr incidence rate from 15% to 1.2%.
IV. Post-Processing Techniques: Precision Burr Removal Solutions for Medical Grade
-
Dry Ice Blasting Cleaning
Using -78℃ dry ice particles at a speed of 300m/s for surface impact is suitable for micro-hole structures (φ0.2mm and above). In the production of artificial joint prostheses, this technology reduced the surface roughness from Ra3.2μm to Ra0.8μm without secondary pollution.
-
Electropolishing Technology
For stainless steel medical components, using a phosphoric acid-based electrolyte at 12V for 3 minutes can reduce the burr height from 0.1mm to 0.01mm. A case study in the production of surgical blades showed that this technology improved blade sharpness by 40%.
-
Laser Micro-Machining
Using femtosecond laser (pulse width 300fs) for selective ablation is suitable for precision medical catheters (ID0.5mm). In the production of neurointerventional catheters, this technology reduced the burr incidence rate from 12% to 0.5% while maintaining material biocompatibility.
V. Quality Control: Establishing Medical-Grade Inspection Standards
-
3D Optical Inspection
Using structured light scanning technology can detect burrs as small as 0.005mm. A case study in the production of pacemaker housings showed that this technology reduced the missed detection rate from 3% to 0.1%.
-
CT Non-Destructive Testing
For complex internal structures, it is recommended to use micro-focus CT (resolution ≤5μm). A case study in the production of cochlear implants showed that this technology can detect hidden burrs, improving product qualification rate to 99.97%.
-
Biocompatibility Verification
After burr removal, it is necessary to conduct testing according to ISO 10993 standards, including 12 indicators such as cytotoxicity and sensitization. A case study in the production of implantable medical devices showed that this verification reduced the clinical adverse reaction rate from 0.8% to 0.02%.
Conclusion
Controlling burrs in medical injection molding is a systematic project that requires starting from mold design and forming a closed-loop management system through precise control of process parameters, matching of material properties, innovation in post-processing techniques, and establishment of a quality control system. As the medical industry continues to raise requirements for product precision, intelligent burr detection and removal technologies will become future development directions. For example, AI vision inspection systems combined with adaptive robotic burr removal can increase production efficiency by 300% and improve product qualification rate beyond 99.99%.











 Home
Home
