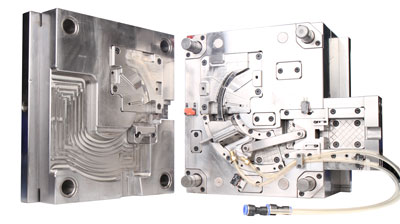
Medical molds, as the core tools in precision manufacturing, directly determine product quality, safety, and production efficiency. Against the backdrop of surging demand for medical devices, multi-cavity mold technology has emerged as a key solution to enhance productivity and reduce costs by enabling the simultaneous injection molding of multiple products in a single cycle. However, the stringent requirements for high precision, cleanliness, and reliability in medical molds pose multiple challenges in multi-cavity design, including runner balance, cooling efficiency, and material compatibility. This article systematically analyzes the core considerations in medical multi-cavity mold design, integrating industry case studies and technical principles.
1. Runner System: The Core Balance Between Flow and Pressure
The runner design of multi-cavity molds must integrate geometric symmetry with rheological principles. For example, in an "eight-cavity" mold, while traditional H-type balanced runners achieve natural balance through equal path lengths, they suffer from long runner lengths and significant pressure losses. Modern designs often employ rheological balance, adjusting runner diameters at each level to compensate for pressure differences. For instance, an electronics connector manufacturer increased the main runner diameter from φ6mm to φ8mm and progressively decreased it (D1>D2>D3). Coupled with Moldflow simulations to optimize branch angles, the weight variation of products was reduced from ±1.2g to ±0.3g.
2. Cooling System: The Invisible Driver of Efficiency and Precision
Medical molds demand stringent deformation control, making traditional straight-through cooling channels inadequate. A medical packaging enterprise adopted a double-layer runner plate design with 3D-printed conformal cooling channels, achieving uniform filling in a 16-cavity mold. This design reduced the runner plate thickness by 50%, cooling surface area by 30%, and injection temperature by 10°C. Key principles include:
-
Independent circuits: Each cavity is equipped with an independent cooling circuit to avoid "water competition."
-
Zonal temperature control: High-gloss areas are separately temperature-controlled to balance shrinkage stresses.
-
Flow rate matching: Runner diameters and flow rates are optimized through CFD simulations to ensure uniform heat exchange.

3. Ejection System: The Ultimate Challenge of Synchronization and Anti-Sticking
The synchronization of ejection in multi-cavity molds directly impacts product yield. A bottle cap mold achieved eight-cavity synchronization through:
-
Guide posts + balance blocks: The ejector plate uses four sets of balance guide posts with tolerances controlled within ±0.015mm.
-
Venting slot design: Ejector pins incorporate 0.05mm-deep venting slots to prevent vacuum adhesion.
-
Segmented ejection: A hydraulic cylinder-driven segmented ejection mechanism reduces ejection force fluctuations.
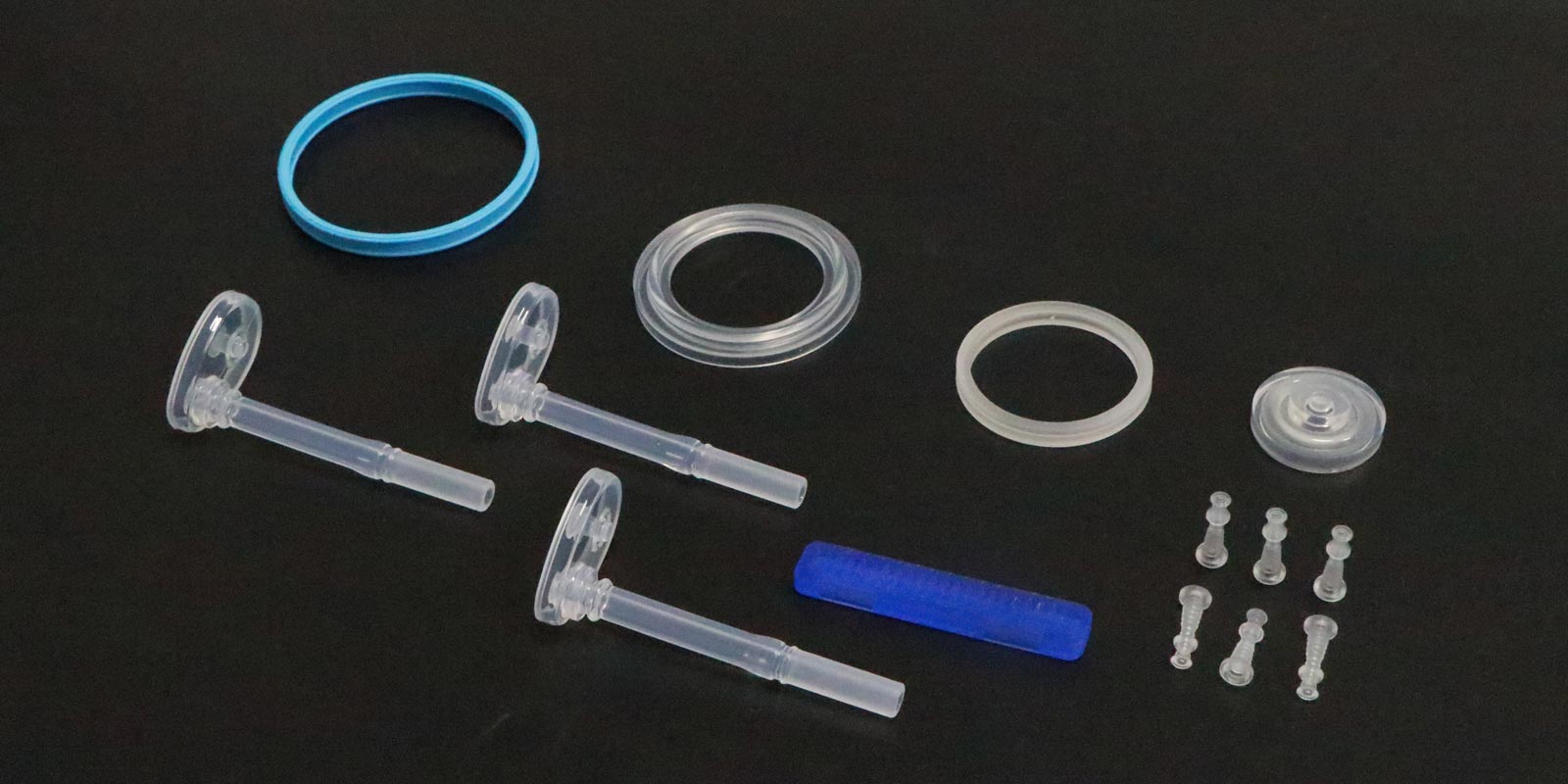
For anti-sticking and surface treatment, medical molds must meet high cleanliness standards, with surface roughness below Ra0.2μm. A syringe piston mold employed PIM (Physical Vapor Deposition) to form a diamond-like carbon (DLC) coating on the cavity surface, reducing the friction coefficient by 60% and ejection force by 40%.
4. Modularity and Intelligence: The Evolutionary Direction of Future Design
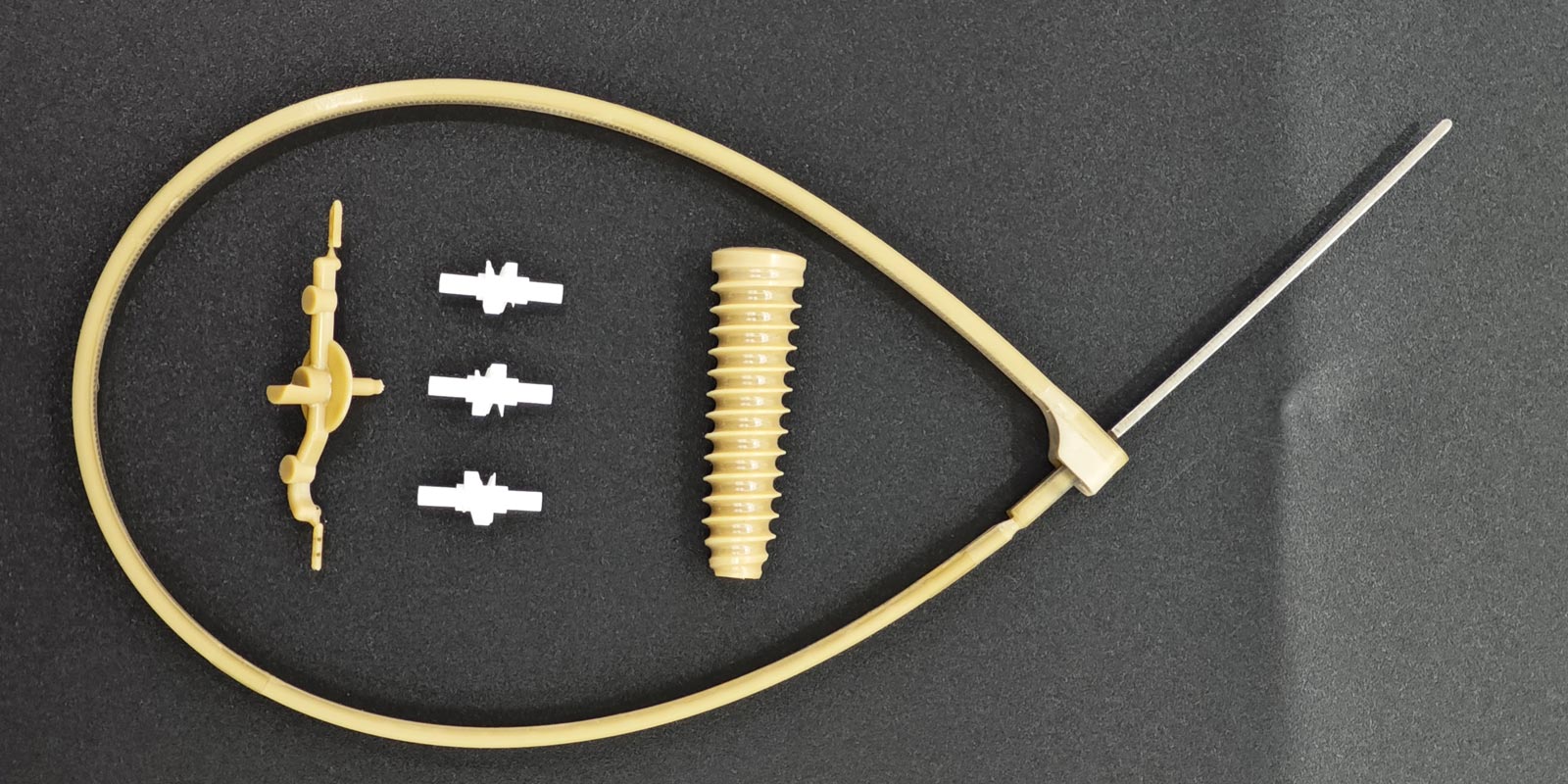
Modular cavity groups enable rapid cavity replacement through standardized interfaces, adapting to multi-variety production. A catheter mold adopted a "core cavity + replaceable inserts" structure, allowing only 10% of mold components to be replaced during product switches, reducing mold change time from 8 hours to 1.5 hours.
AI-assisted design and IoT monitoring leverage AI algorithms to automatically generate balanced runner solutions based on 3D product models, optimizing gate positions and sizes. An enterprise used an IoT system to monitor mold temperature, pressure, and ejection forces in real time, predicting maintenance nodes and extending mold life to 1.5 million cycles.











 Home
Home
